Atomic Number and Mass Number
Atomic Number and Mass Number: Overview
This topic covers concepts, such as, Atomic Number and Mass Number, Atomic Numbers, Properties of Isotopes & Properties of Isobars etc.
Important Questions on Atomic Number and Mass Number
and are isotopes while and are isobars.
The mass of positively charged particles present in an atom is found to be times that of an electron. Identify the element and write its electronic configuration.
Which of the following options shows the correct set of atomic numbers and valency for element X in a neutral state as given below?
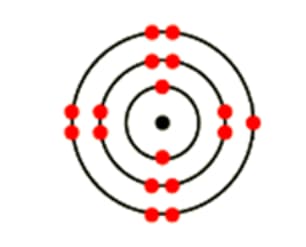
What is the atomic mass of gold?
A metal (mass number ) having same number of protons and neutrons, combines with two chlorine atoms. Identify the element with which electronic configuration of this metal matches in combined state.
What is the atomic number of iron?
What is the atomic number of helium?
Give the atomic weights of Zinc and Copper.
Find the number of electrons, protons and neutrons in .
Isobars are atomic species that have the same _____ number, but a different atomic number.
Properties of Isobars: Isobars of an element have the same number of _____ .
Why isobars have different physical properties?
What are the properties of isobars?
If you know the mass number and the atomic number of an atom, how might you calculate its number of neutrons?
The atoms of any element have the same atomic number but a different mass number are called -
Write the definition of mass number.
Write the definition of atomic number.
Write the names of three isotopes of hydrogen.
The nucleus of an atom has its mass.
